POLICY BRIEF - Powering Protection Locally: The Academic Catalyst for Advancing Vaccine Self-Reliance and Global Equity through Gavi 6.0
Posted 7 months ago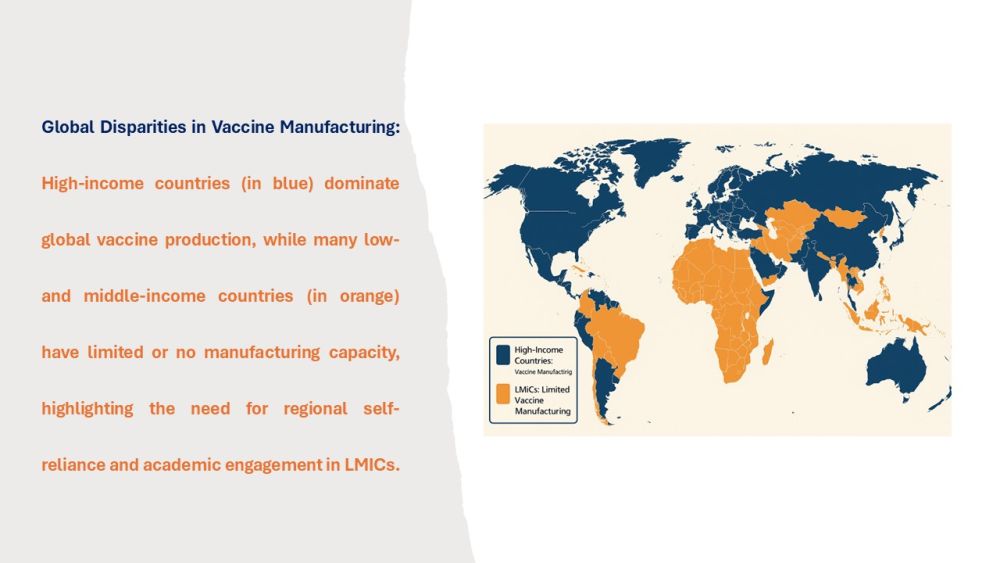
Why Local Vaccine Production Matters?
The coronavirus disease 2019 (COVID-19) pandemic exposed critical vulnerabilities in global health systems, notably the overdependence on a limited number of vaccine manufacturers concentrated in high-income countries. Low and middle-income countries (LMICs), despite facing urgent needs, were often the last to receive access to essential vaccines. This inequity delayed pandemic responses, exacerbated public health crises, and underscored the pressing need for regional self-sufficiency in vaccine production.
GAVI 6.0 (2026 - 2030), the Vaccine Alliance’s strategic framework (previously Global Alliance for Vaccines and Immunization), emphasizes country-led, sustainable, and equitable vaccine access as core principles. Local vaccine production directly supports these goals by building in-country capacity, reducing supply chain disruptions, enhancing national security, and accelerating responses to outbreaks, epidemics, and pandemics. Academic and research institutions are essential partners in this effort, acting as innovation incubators, talent hubs, and policy advisors, contributing directly to the development of resilient, inclusive, and adaptive immunization systems.
Strategic Context: Gavi 6.0 and the Role of Academia
Gavi 6.0 sets forth four strategic goals:
- Introduce and scale up vaccines
- Strengthening health systems to increase equity in immunization
- Improve programmatic and financial sustainability
- Ensure healthy markets for vaccines and related products
To support these goals, Gavi identifies ten guiding principles that define its values and approach, including being country-led and sustainable, community-owned and inclusive, focused on zero-dose and missed communities, gender-sensitive, fragility-responsive, integrated, resilient, climate-sensitive, innovative, and collaborative and accountable.
Academic institutions in Pakistan and likewise in other countries can significantly contribute to these objectives by facilitating local vaccine production, which not only improves supply chain independence but also fosters innovation, strengthens national health security, and enhances regional collaboration.
Policy Recommendations: A Framework for Academic Engagement in Local Vaccine Production
To maximize their contribution to Gavi 6.0, academic and research institutions in LMICs should adopt a structured six-pillar framework for supporting local vaccine production:
1. Human Capital Development and Workforce Training
Building a sustainable local vaccine manufacturing ecosystem requires a specialized, well-trained workforce. Academic institutions must:
- Develop graduate and post-graduate curricula in fields such as vaccinology, molecular biology, pharmaceutical engineering, regulatory science, and biotechnology.
- Create partnerships with international universities and centers of excellence to provide joint degrees, exchange programs, and capacity-building fellowships.
- Establish hands-on biomanufacturing labs and simulation training centers to train scientists, technicians, and quality assurance professionals in real-world vaccine production processes.
This directly supports Gavi’s principles of country-led, sustainable, and integrated systems by building long-term national capacity for self-reliant immunization infrastructure.
2. Research and Development Hubs for Regionally Relevant Vaccines
Academic institutions can drive context-specific innovation by focusing on diseases endemic to their regions (e.g., Lassa fever in West Africa, Chikungunya in South Asia, or Human Papillomavirus (HPV) globally).
- Establish vaccine innovation centers that focus on preclinical and clinical development of locally relevant vaccines.
- Support research on thermostable formulations, needle-free delivery systems, and other innovations suited to low-resource settings.
- Apply for international grants (e.g., CEPI, PATH, Gavi) to co-develop and scale region-specific candidates in collaboration with biotech firms.
This supports Gavi’s commitment to innovation, adaptability, and reaching zero-dose and missed communities with solutions tailored to their unique challenges.
3. Regulatory Science and Policy Support
Delays in vaccine approval and rollout are often tied to underdeveloped regulatory systems. Universities can:
- Conduct research on streamlining national and regional regulatory pathways.
- Offer technical support to national regulatory authorities (NRAs) through policy analysis, capacity assessments, and training programs.
- Advocate for harmonized standards across regional blocs (e.g., African Medicines Agency, Association of Southeast Asian Nations (ASEAN) to enable cross-border vaccine trade .
Academic research in regulatory science also contributes to Gavi’s goals for healthy markets, resilient systems, and collaborative governance.
4. Feasibility and Market Analysis for Local Production
Determining the viability of local manufacturing requires deep technical and economic analysis. Academic institutions can:
- Conduct cost-benefit and return-on-investment studies for domestic vaccine production.
- Analyze infrastructure gaps, supply chain needs, and technology transfer readiness.
- Publish findings to inform government investment strategies and attract private sector engagement.
These activities support financial sustainability and Gavi’s broader goal to develop equitable and functioning vaccine markets.
5. Multi-Sectoral Collaboration and Public-Private Partnerships
Universities should act as conveners of stakeholders in the vaccine production ecosystem by:
- Forming consortia with government health ministries, biotech companies, international donors, and NGOs.
- Coordinating research and technology transfer projects with multinational firms under voluntary licensing or co-manufacturing models.
- Hosting public forums, innovation fairs, and policy dialogues to align vaccine production efforts with national immunization strategies.
This directly addresses Gavi’s principles of being collaborative, accountable, and inclusive, encouraging shared ownership of vaccine equity goals.
6. Community Engagement and Ethical Governance
Local vaccine production must be aligned with community needs and values. Academic institutions must:
- Conduct social science and bioethics research to assess community acceptance of locally produced vaccines.
- Facilitate participatory design processes to ensure vaccines meet cultural, religious, and gender-related preferences.
- Establish ethical review boards and transparency mechanisms to maintain public trust and accountability.
This supports Gavi’s principles of community ownership, gender-focus, and inclusive public health governance.
7. Impact Potential: Why This Matters
If implemented, academic engagement in local vaccine production could:
- Reduce dependence on external suppliers during health emergencies.
- Enable faster outbreak response and pandemic preparedness.
- Tailor vaccines to region-specific disease burdens and demographic needs.
- Strengthen regional economies through job creation and scientific advancement.
- Improve public trust in vaccination by boosting national pride and accountability.
Local production, when embedded in academic ecosystems, transforms immunization into a knowledge-driven, community-empowered effort aligned with long-term health sovereignty.
8. An Urgent Call to Action
The coming decade presents a unique opportunity to reimagine global vaccine equity. Gavi 6.0 has laid a bold blueprint centered on equity, innovation, and sustainability. Academic institutions must now rise to the challenge, not just as educators or researchers, but as co-builders of a more just and resilient immunization landscape.
By adopting the six-pillar framework outlined in this brief, universities and research institutions can become engines of regional vaccine self-reliance helping countries not only immunise their people but also manufacture their future.
The time to power protection locally is now.
Author's Brief Biosketch
Dr. Gul Shahnaz is a tenured Professor of Pharmacy at Quaid-i-Azam University, Pakistan. Her research focuses on innovative drug delivery systems for infectious diseases and cancer. With over 80 publications, she specializes in nanocarriers, inhalers, and microneedle technologies, advancing therapeutic strategies and translational pharmaceutics.
Institutional Affiliation:
Department of Pharmacy, Quaid-i-Azam University, Islamabad, Pakistan.





